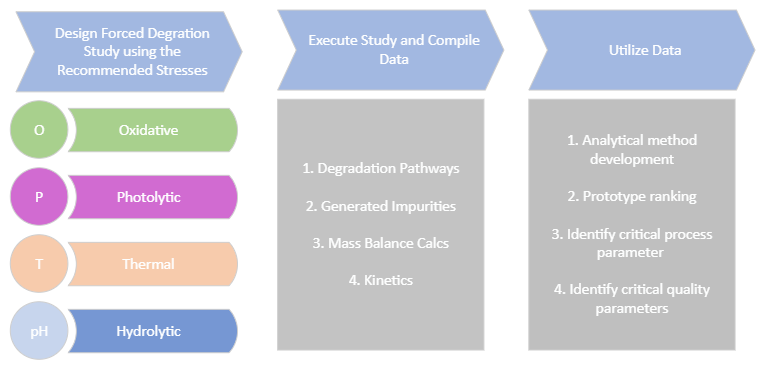
Design and conduct stress test studies in accordance with ICH requirements for drug substances and drug products. These forced degradation studies allow for the development of stability indicating analytical methods that are used in the general assay and impurities method in formal stability studies. These studies should be performed and must be part of regulatory submissions.
Conduct forced degradation studies to understand drug/excipient compatibility and guide formulation development, manufacturing process and storage conditions of the product.
Products are exposed to oxidative, photolytic, thermal and pH ranged hydrolytic stresses as per the experimental design. A target of 5 to 20% degradation for the active ingredient is intended. Combinations of stressors can also be applied in the study design to promote greater degradation or align with known process related parameters